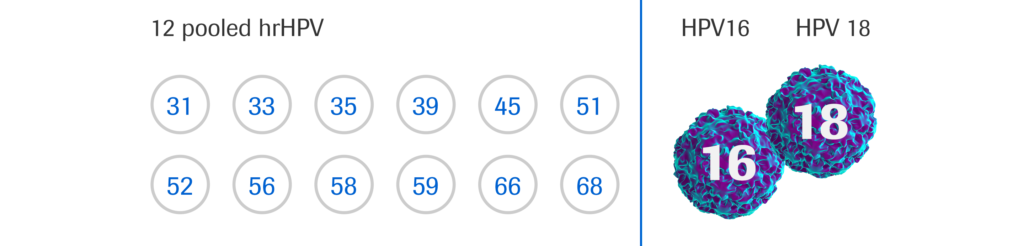
HPV-16 | HPV-18 | 12 tipes

+ß-globin
AmpErase enzyme
Cervical cancer rarely causes overt symptoms in its early stages — when treatment is most effective — so screening for the types of HPV infection at the greatest risk of progressing to cervical pre-cancer and cancer is imperative.
The cobas® HPV test is clinically validated and FDA-approved to provide individual results for HPV 16 and HPV 18, along with a simultaneous, pooled result for other high-risk genotypes, all in one run, from one patient sample. By providing 3-in-1 results, clinicians are able to better risk stratify patients, and make patient management decisions with confidence.
Benefits and Features
Both cobas® 4800 HPV test and the cobas® HPV test for use on cobas® 68/8800 systems are clinically validated and FDA approved. They utilize amplification of target DNA by the polymerase chain reaction (PCR) and nucleic acid hybridization for the detection of 14 high-risk HPV (hrHPV) types in a single analysis. HPV DNA tests have extensive longitudinal data to support the safety of a negative result and to ensure confidence in a negative result, each cobas® HPV test also includes appropriate controls to verify human cells are present in the sample.
Results you can trust by our built-in quality & safety features
▸ Internal control: The ß-globin internal cellular control helps prevent false negatives. HPV negative specimens with a negative ß-globin result are flagged as invalid, helping to prevent reporting of false negative results.
▸ Use of AmpErase enzyme: Each reaction contains AmpErase enzyme, reducing the risk of false positive results from carry-over contamination by differentiating amplification products from target molecules.
Peace of mind patients deserve
▸ All Roche assays are validated in large, clinical studies that evaluate specific product performance in various screening strategies (e.g., cobas® HPV and cobas® 4800 Systems in the ATHENA trial, and cobas® HPV for use on the cobas® 6800/8800 Systems and triage with CINtec® PLUS Cytology in the IMPACT trial).
▸ Validated for detection of >CIN2 lesions and not simply presence of HPV
▸ Validated to the standards




