Troponin T test from Roche is approved by FDA
Breakthrough development for Americans with suspected heart attack, as next generation Troponin T test from Roche is approved by FDA.
The 510(k) clearance of Elecsys® Troponin T Gen 5 STAT (TnT Gen 5 STAT) assay has been eagerly awaited in the USA for many years and will now help their doctors to effectively diagnose and manage patients with a suspected heart attack, also called Myocardial Infarction (MI).
With this launch, Roche becomes the first in vitro diagnostics company to receive FDA clearance for a truly high sensitive Troponin T assay. This follows on from the TnT Gen 5 STAT (named Troponin T-high sensitive in the rest of the world) receiving CE Mark launch in 2009.
 Every minute counts
Every minute counts
Every 43 seconds1, a person in the U. S. will have a heart attack. A heart attack, also termed acute myocardial infarction (AMI), is a cardiac event in which the blood supply to an area of the heart muscle is interrupted. This causes the cardiac muscle cells to die and to release Troponin into the blood stream. Patients with chest pain and suspected AMI account for approximately 8 million2 of all emergency room consultations in the U.S. Only a fraction of them (5-20%) have an actual AMI, so a fast diagnosis is critical for proper patient treatment and hospital resource management.
A more sensitive test is better for patients
Accurate diagnosis of AMI requires sensitive diagnostic tests that can detect Troponin release early and allow doctors to make faster decisions for their patients. Early diagnosis and initiation of treatment greatly impacts outcome and can save lives.
The new generation Troponin T test from Roche Diagnostics has a much higher sensitivity to detect AMI than previous versions of the test. In conjunction with the rapid turnaround time of 9 minutes, this accelerates decision-making and the higher sensitivity also improves detection of smaller infarctions. Together these features of the test maximize the potential for more effective treatment.
Robert H. Christenson, Ph.D., D(ABCC), FACB, Professor of Pathology, Professor of Medical and Research Technology, Medical Director, Point of Care Services at the University of Maryland Medical Center commented,
“Availability of high-sensitivity cardiac Troponin T is a significant step forward for both better healthcare and resource utilization. High sensitive TnT availability will allow earlier MI diagnosis and risk assessment, as well as more timely rule-out of MI so patients can placed on appropriate care path more efficiently.”
A useful tool for doctors
This more sensitive test has been rapidly adopted by doctors and its clinical utility is supported by more than 600 peer-reviewed publications. The European Society of Cardiology 2015 guidelines confirm that this test allows a faster diagnosis of AMI and recommends its use for quicker decisions.3
Prof. Christian Müller about high sensitivity cardiac test
With such compelling clinical utilities and the automation advantages associated with Roche Diagnostic solutions, the Elecsys TnT Gen 5 test can provide the diagnostic confidence that healthcare professionals need to diagnose and manage patients in emergency settings and beyond.
Roche Leadership in Cardiac Testing
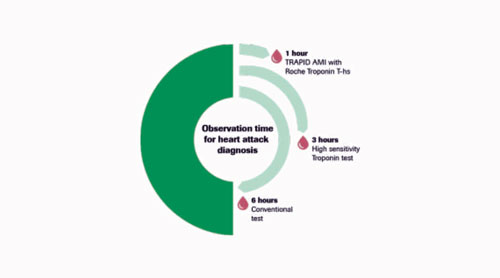
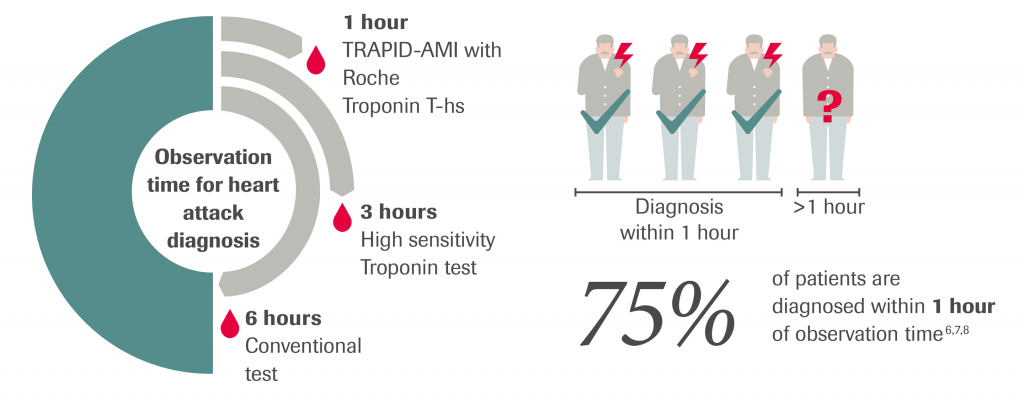
Roche is the world leader in cardiac diagnostics, touching more patients’ lives than any other company revolutionizing the care of life-threatening conditions like myocardial infarction and heart failure by bringing tests such as Troponin T, Troponin T – high sensitive and NT-proBNP into wider use in the clinic. Roche does not just invest in developing new tests, but also supports groundbreaking research that advances the use of these tests in clinical practice. One such example is the study TRAPID-AMI – .the results of this trial show that the Troponin T-high sensitive test can give a safe and rapid diagnosis of AMI within a 1-hour observation time which is faster than the 3-6 hours using the conventional diagnostic approach. As well as supporting groundbreaking clinical trials, such as TRAPID-AMI, Roche Diagnostics continues to innovate with research into tests like Growth Differentiation Factor-15 (GDF-15), a stress responsive cytokine. A GDF-15 level integrates information from both cardiac and extra-cardiac disease pathways and predicts adverse outcomes, independent of established markers, thereby demonstrating its paramount prognostic power.
source: http://www.cobas.com